- Centro Interuniversitario per la Promozione dei Principi delle 3R nella Didattica e nella Ricerca
An Innovative In Vitro Open‐Angle Glaucoma Model (IVOM) Shows Changes Induced by Increased Ocular Pressure and Oxidative Stress
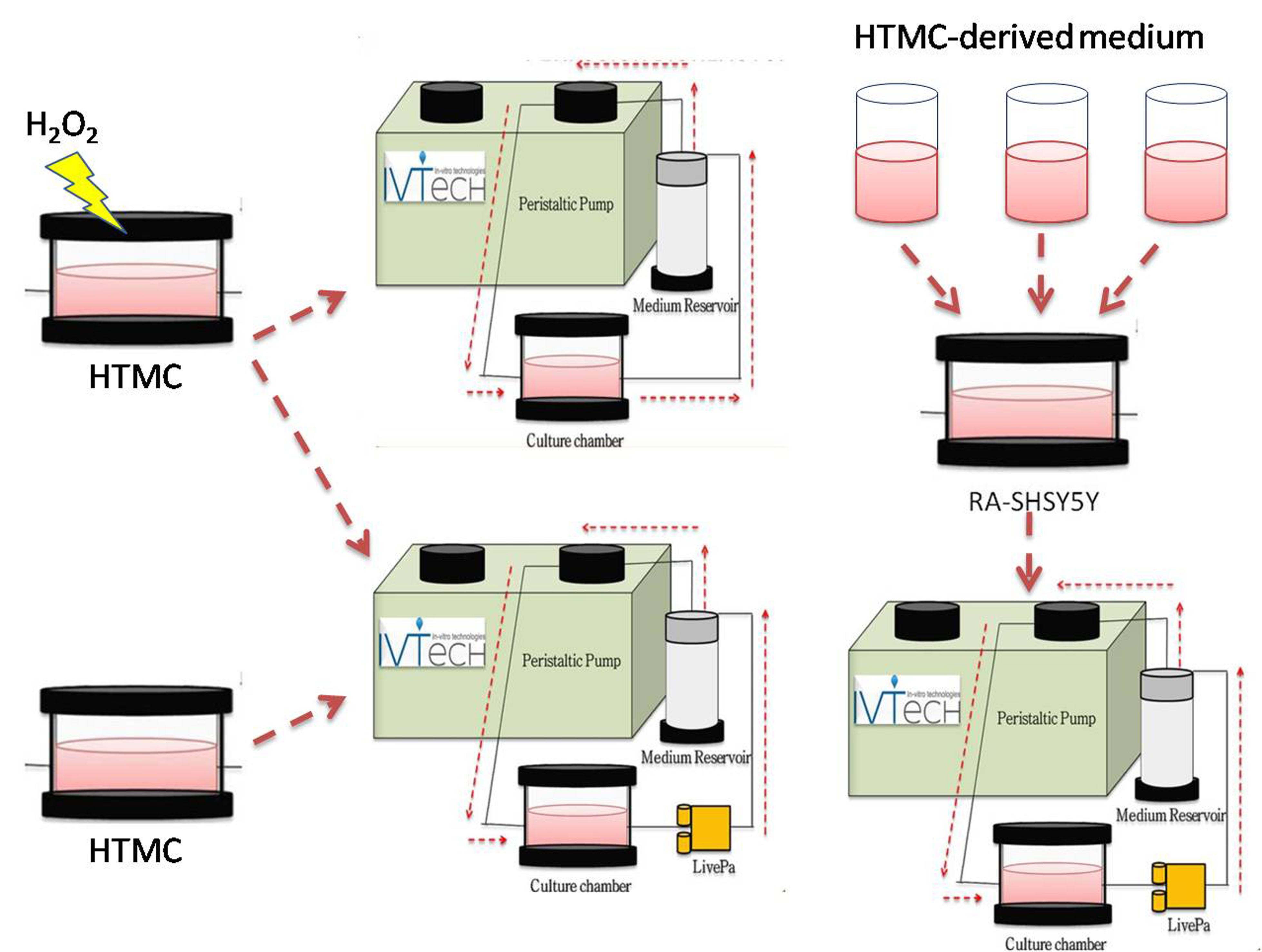
Primary Open‐Angle Glaucoma (POAG) is a neurodegenerative disease, and its clinical outcomes lead to visual field constriction and blindness. POAG’s etiology is very complex and its
pathogenesis is mainly explained through both mechanical and vascular theories. The trabecular meshwork (TM), the most sensitive tissue of the eye anterior segment to oxidative stress (OS), is the
main tissue involved in early‐stage POAG, characterized by an increase in pressure. Preclinical assessments of neuroprotective drugs on animal models have not always shown correspondence
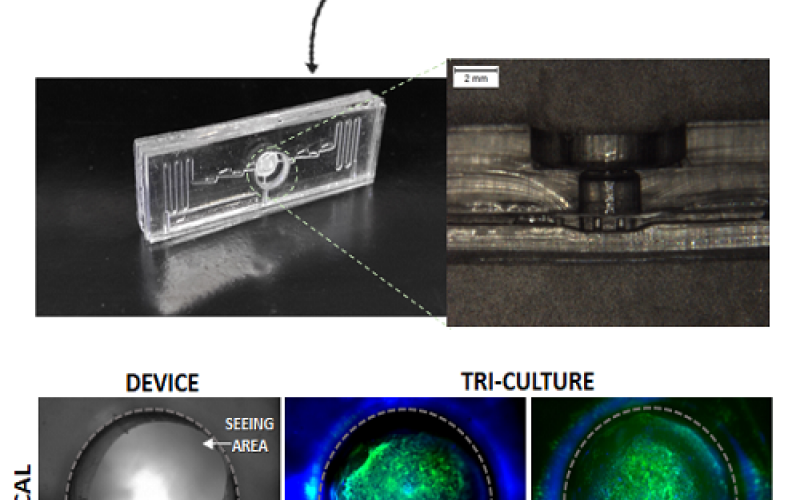
with human clinical studies. In addition, intra‐ocular pressure management after a glaucoma diagnosis does not always prevent blindness. Recently, we have been developing an innovative in vitro 3Dadvanced human trabecular cell model on a millifluidicplatform as a tool to improve glaucoma studies. Herein, we analyze the effects of prolonged increased pressure alone and, in
association with OS, on such in vitro platform. Moreover, we verify whethersuch damaged TM triggers apoptosis on neuron‐like cells. The preliminary results show that TM cells are less sensitive
to pressure elevation than OS, and OS‐damaging effects were worsened by the pressure increase.
The stressed TM releases harmful signals,which increase apoptosis stimuli on neuron‐like cells, suggesting its pivotal role in the glaucoma cascade.
| Allegato | Dimensione |
|---|---|
| 1.89 MB |