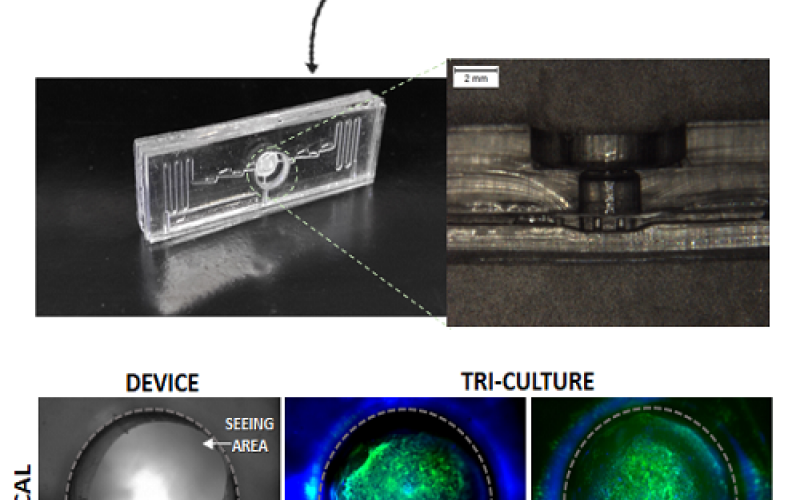
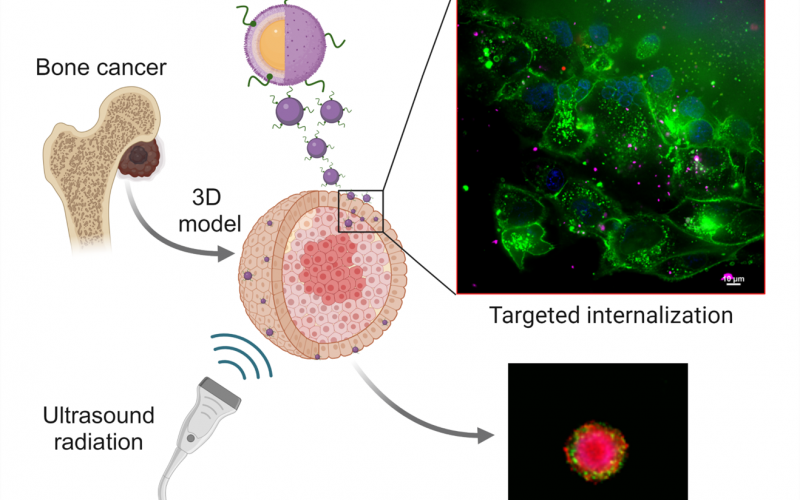
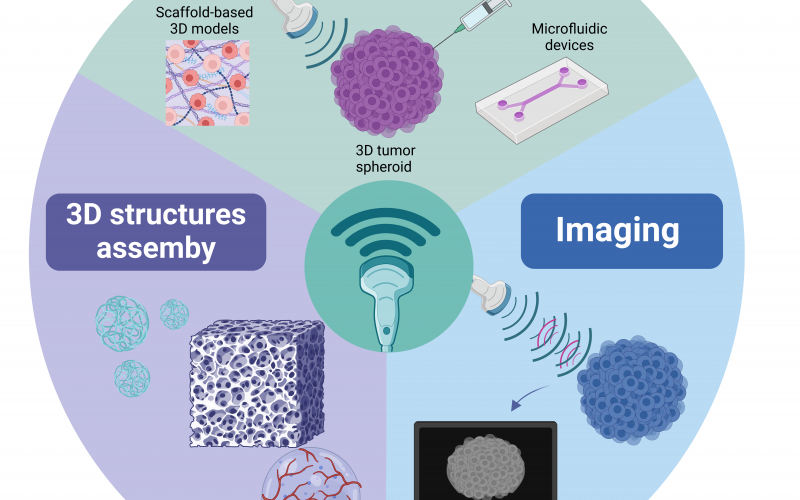
- Centro Interuniversitario per la Promozione dei Principi delle 3R nella Didattica e nella Ricerca
The Depolarization-Evoked, Ca2+-Dependent Release of Exosomes From Mouse Cortical Nerve Endings: New Insights Into Synaptic Transmission
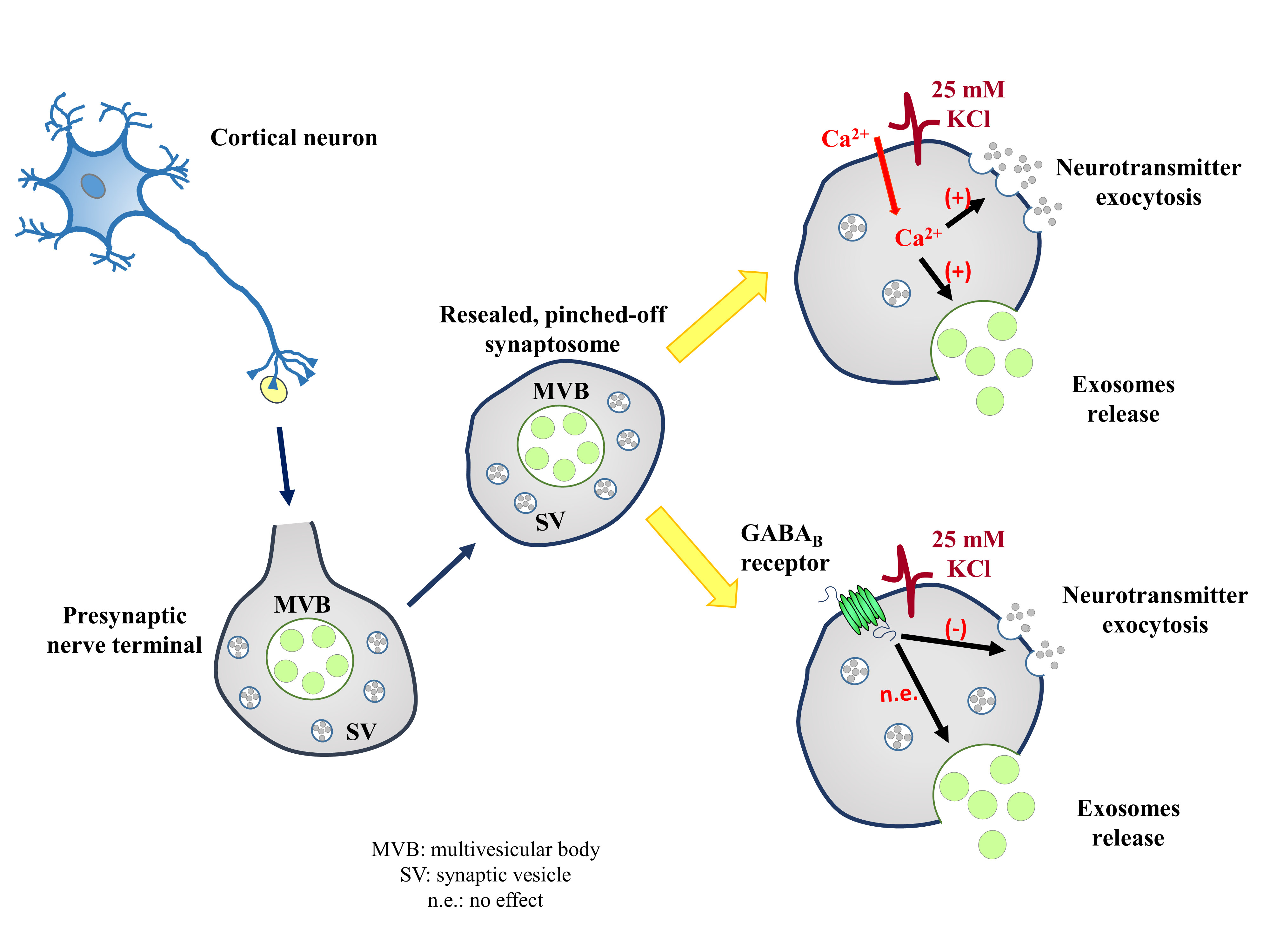
Whether exosomes can be actively released from presynaptic nerve terminals is a matter of debate. To address the point, mouse cortical synaptosomes were incubated under basal and depolarizing (25mMKCl-enriched medium) conditions, and extracellular vesicles were isolated from the synaptosomal supernatants to be characterized by dynamic light scattering, transmission electron microscopy, Western blot, and flow cytometry analyses. The structural and biochemical analysis unveiled that supernatants contain vesicles that have the size and the shape of exosomes, which were immunopositive for the exosomal markers TSG101, flotillin-1, CD63, and CD9. The marker content increased upon the exposure of nerve terminals to the high-KCl stimulus, consistent with an active release of the exosomes from the depolarized synaptosomes. High KCl-induced depolarization elicits the Ca2+-dependent exocytosis of glutamate. Interestingly, the depolarization-evoked release of exosomes from cortical synaptosomes also occurred in a Ca2+-dependent fashion, since the TSG101, CD63, and CD9 contents in the exosomal fraction isolated from supernatants of depolarized synaptosomes were significantly reduced when omitting external Ca2+ ions. Differently, (±)-baclofen (10 μM), which significantly reduced the glutamate exocytosis, did not affect the amount of exosomal markers, suggesting that the GABAB-mediated mechanism does not control the exosome release. Our findings suggest that the exposure of synaptosomes to a depolarizing stimulus elicits a presynaptic release of exosomes that occurs in a Ca2+-dependent fashion. The insensitivity to the presynaptic GABAB receptors, however, leaves open the question on whether the release of exosomes could be a druggable target for new therapeutic intervention for the cure of synaptopathies.
| Attachment | Size |
|---|---|
| 1.9 MB |